|
Medical
Device & Diagnostic Industry Magazine
MDDI
Article Index
Originally Published MDDI
July 2005
Cover Story
MD&DI’s Top Ten
technologies
MD&DI
identifies and explores the Top 10 medical device
technologies that drive industry today—and the
applications that use them.
In an industry with so many innovations leading to so
many radical improvements in patient care, it is no easy
task to determine 10 top application areas and
technologies. But we at MD&DI decided to
give it a try anyway, to showcase the sectors with the
most important developments affecting patient care,
clinical practice, product creation, and the device
industry as a whole. Of course, this list does not mean
that other areas are less important, but we feel these
10—although in no particular order—are having a bigger
effect at this time.
What constitutes a top 10
technology area? There is no set definition. Rather, we
set out to find sectors with new products making
significant contributions to healthcare. We looked for
transformative innovation that was affecting the
industry right now. Hence, futuristic areas like
nanotechnology aren’t on this list—but don’t be
surprised if they make the cut a few years from now.
You may not necessarily agree that these are the
top 10 medical technologies. But we hope you’ll agree
that they are important and worthy of recognition.
Implantable
Elution Devices: Reshaping the Industry
Carotid
Artery Stents: A Step Toward Preventing
Strokes
Heart
Assist Devices: Keeping Patients’ Interest at
Heart
Artificial
Bone and Skin Grafts: New Materials Provide Better
Scaffolding
Artificial
Orthopedic Disks: Flexible Disks Imitate
Vertebrae
Nucleic
Acid–Based IVDs: Diagnoses in a Day, Not Weeks
Medical
Lasers: The Wavelength of the Future
Medical
Imaging Technology: The Value Beneath the Surface
Wireless
Technology: Hospitals and Homes Go Unplugged
Computer-Assisted
Surgery: The Digital
OR
Implantable Elution Devices: Reshaping the
Industry
Erik Swain
 |
| Boston Scientific leveraged
outside suppliers and vendors to ensure the Taxus
eluted accurately and
effectively. |
Since 2003, it’s been impossible to discuss the state
of the medical device industry without delving into
drug-eluting stents. For patients, clinicians,
companies, and investors, these stents have brought
benefits of a magnitude rarely seen in the device
sector. As new generations are developed, and elution
principles are applied to other technologies, their
tremendous effect should continue for the foreseeable
future. Some consider the two FDA-approved drug-eluting
stents—Cypher from Johnson & Johnson’s
Cordis Corp. (Miami) and Taxus from
Boston Scientific Corp. (Natick, MA)—to
be the first blockbuster devices.
“This is part
of a progression of technologies dealing with coronary
artery disease,” says Michael Drues, PhD, president of
Vascular Sciences (North Grafton, MA).
“First there was bypass surgery, then angioplasty, then
stents, and now there are drug-eluting stents. In
general, each one has given better clinical outcomes
than its predecessor.”
The clinical benefit has
come in the reduction of restenosis rates. As many as
one-third of patients receiving bare-metal stents suffer
from reblockage of the artery. Drug-eluting stents
reduce the inflammation caused by the stent pressing
against the artery, dropping restenosis rates into
single digits.
This benefit caused “a massive
shift in the market in a very short period of time,”
says Thomas Gunderson, managing director and senior
research analyst for Piper Jaffray
(Minneapolis). “It went from four or five companies
selling bare-metal stents to two selling drug-coated
stents, which soon accounted for more than 80% of the
market. It was quickly adopted by clinicians because it
was a way to do the same procedure while reducing
complications.”
The development of drug-eluting
stents also significantly affected industry. For one, it
signaled the emerging importance of combination
products. Henceforth, device companies need to consider
whether a device combined with a drug or biologic will
provide a better clinical outcome. If so, that means
they need to figure out how to work with other branches
of FDA aside from CDRH.
Drug-eluting stents have
also changed the way device companies work with the
Centers for Medicare and Medicaid
Services (CMS; Baltimore). Traditionally,
device companies hadn’t begun the CMS coverage process
until after FDA approval. But J&J worked with CMS to
secure coverage, coding, and payment for Cypher before
obtaining FDA approval, allowing for reimbursement as
soon as the product hit the market. This opened a new
level of communication between CMS and industry. Of
course, it helped that J&J and Boston Scientific
were willing to do outcomes research up front. Whether
other firms will be expected to do the same, and whether
they can afford it, remain to be seen.
Although
the technological challenges were tremendous, J&J
and Boston Scientific found partners in other
disciplines who could help overcome those challenges.
For example, after J&J came up with the idea for a
drug-eluting stent, it went to SurModics
Inc. (Eden Prairie, MN), for help with
developing a coating. “We said we thought we had a
technology that could be useful, and that began a
process of years of testing,” says David Wood, general
manager of SurModics’ drug-delivery business. Both
J&J and Boston Scientific were able to leverage
these kinds of outside resources to develop coatings
that would stay on the stent, properly contain the drug,
be compatible with the human body, and elute the drug at
the correct rate over the correct period of time. The
difficulty of this task is evidenced by what’s happened
to their competitors: some have failed, and others lag
behind because they’ve not been able to get the coating
right.
 |
| J&J worked with CMS to
secure reimbursement for its Cypher stent before
obtaining FDA
approval. | The
manufacturing challenges have been equally daunting, and
both firms have had setbacks. Making the product
requires an extraordinary level of precision and a great
need for consistency, the likes of which much of the
device industry has not seen before. For example,
vendors such as Machine Solutions Inc. (Flagstaff, AZ)
had to make their crimping process even more precise
than before to prevent the risk of upsetting the drug
and coating.
What does the future hold? New stent
and polymer designs, both from the current players and
from companies not yet in the U.S. market, should enable
drug-eluting stents to be used in riskier patient
populations.
“There will be systems developed
that include biodegradable polymers and stents,” says
Wood. “These might affect vulnerable plaque, which for
the most part is an untreated problem now. But that’s
yet to be demonstrated on any sort of clinical
model.”
And eventually, we could see multiple
substances eluted from one stent, or elution being used
on other kinds of devices.
“In my opinion,
drug-eluting stents are important to the extent that
they take us to the next step: biologics,” says Drues.
“Drugs are good for certain things, such as containing
thrombus and inflammation. But they are not particularly
good at containing mitosis, or cell division, which is a
major cause of restenosis. Biologics, on the other hand,
can control the nucleus of the cell. For example, they
have been shown to control mitosis and hyperplasia in
cancer cells. That means we might be able to use
biologics to turn off hyperplasia. Ideally, we’d like to
be able to elute both drugs and biologics, to use the
drugs for what they do best and the biologics for what
they do best. When you get sick, the doctor may give you
prescriptions for two to four medications. Why can’t it
be the same for devices? What if we could have a stent
carrying two drugs and two biologics, all able to do
different things to control the problem?”
Carotid Artery Stents: A Step Toward
Preventing Strokes
 |
| The Acculink uses a rapid
exchange platform that offers device control,
faster exchanges, and ease of use.
|
Erik Swain
As cardiovascular stents revolutionized treatment of
coronary artery disease, so could carotid artery stents
transform stroke prevention. In theory, carotid stenting
and distal protection could supplant carotid endarectomy
for some patients at risk of stroke. After all, coronary
stenting supplanted bypass surgery for certain patients
at risk of heart attack. In practice, it remains to be
seen whether it will play out that way. But the 2004 FDA
approval of Guidant Corp.’s Acculink carotid stenting
system and Accunet embolic protection system gives U.S.
clinicians a chance to find out.
Carotid
endarectomy is a highly invasive procedure in which
plaque is removed from a patient’s carotid arteries
after an incision through the neck. By contrast, in the
stenting procedure, the stent is inserted into a small
puncture in the groin and then moved into position. It
has emerged as a much-needed alternative for patients
considered too great a risk for surgery.
But is
the minimally invasive procedure going to be the
preferred option?
“You have an extremely
successful open surgery, and now the supposed solution
is to do it intravenously with a different doctor, so
that could lead to turf wars,” says Thomas Gunderson,
managing director and senior research analyst for
Piper Jaffray (Minneapolis). “It will
be difficult, in my opinion, to prove that [carotid
artery stenting is] better than a procedure that claims
95% success. The challenge will be training
interventionalists to get up in the neck and brain to
clean the carotids out without debris going
northward.”
Michael Drues, PhD, agrees. Drues is
president of Vascular Sciences (North
Grafton, MA). He says that carotid stenting is a much
more difficult procedure than coronary artery stenting.
If plaque is dislodged into the brain, it could cause
permanent brain damage, whereas if plaque is dislodged
into the heart, permanent damage is less likely. Studies
have shown that quality of life may not be lost when
part of the heart can no longer function, but it may be
lost to a great degree when part of the brain can no
longer function, he says.
“The biggest
competitor may be the status quo,” Drues says. Carotid
endarectomy “is a good procedure for safety and
effectiveness. Can it be made better in a less-invasive
fashion?”
The key is distal protection, which
helps prevent the plaque from traveling to the brain
during the procedure. For Guidant, that means the
performance of the Accunet may make or break the entire
concept.
The design and manufacturing of carotid
stents presents different challenges from coronary
stents. The carotid arteries are much smaller, and it’s
much more difficult to maneuver a device into them.
“Ease of implantation and deliverability are major
challenges,” says David Wood, general manager of the
drug-delivery business for SurModics
Inc. (Eden Prairie, MN). “You need to attach
molecules to the surface of the catheter to make it
slippery enough to maneuver in there. The catheter is
made of a plastic that has quite a bit of
friction—that’s where hydrophilic coatings come
in.”
Clinical trials showed the Guidant system
cut the likelihood of stroke in the blockage area
compared with the conventional surgery. If those results
are borne out in the real world, it could reshape stroke
prevention.
Heart Assist Devices: Keeping Patients’
Interest at Heart
Maria Fontanazza
 |
 |
Top: The St. Jude Epic ICD allows
for heart rate changes
resulting from
activity. Bottom: The left-VAD from Thoratec Inc.
(Pleasanton, CA) is the only VAD approved by FDA
for
permanent
implantation. |
Devices that help the heart to function are one of
the most fruitful areas for patients, caregivers, and
companies. Whether implantable or external, electronic
or mechanical, heart devices are becoming smaller and
easier to implant. They are restoring quality of life to
patients who would have had little hope a few years
ago.
Pacemakers have developed significantly
since their beginnings more than 40 years ago. Changes
made to the leads have enabled implantation without
opening the chest cavity. Lithium iodine batteries have
extended pacemaker life from one year to up to more than
10 years, and new titanium casings have decreased
electromagnetic interference.
The device’s
ability to modify the heart rhythm with a person’s
activity level has made it even easier to maintain a
normal lifestyle. St. Jude Medical (St.
Paul, MN) developed an algorithm for suppressing atrial
fibrillation, which the company has incorporated into
some of its pacemakers and implantable cardioverter
defibrillators (ICDs). The technology’s pacing allows
for heart rate changes resulting from everyday
activities and sleep cycles.
For people who
experience abnormally fast and erratic heartbeats, ICDs
can offer relief. About the size of a pager, ICDs
contain leads that are channeled to a pulse generator,
which is implanted beneath the skin with a battery. When
an ICD detects irregular rhythms, it shocks the heart
back to a normal beat. Like pacemakers, some also record
heart patterns that a doctor can review later.
In 2001, a fairly new system that incorporates
either a defibrillator or pacemaker surfaced in the
United States. Cardiac resynchronization therapy (CRT)
devices restore the heart’s two ventricles to a
simultaneous beat. While a pacemaker has only two leads
for the right atrium and right ventricle, CRTs have a
third lead in a vein on the left ventricle.
“Relative to just a basic defibrillator or
pacemaker, the CRT is targeted for congestive heart
failure patients who have a very poor ejection fraction,
or amount of blood volume that gets out of the heart,”
says Greg Aurand, senior medical devices analyst at
Zacks Investment Research Inc. (Chicago). Poor
synchronization enlarges the heart. CRTs can help shrink
the organ down to a more-normal size.
Other
devices that reduce heart muscle strain are ventricular
assist devices (VADs), which vary in design. VADs
include an energy supply, a control system, and a pump.
For the energy-supply component, some use a battery and
some use air. The control system and energy supply are
external, and the pump can be either internal or
external.
“They’re almost fully implantable
now,” says Aurand. “They used to be partially
implantable. The pump was outside the body and plugged
into a wall, because the patients had no other way of
power-sourcing the implant.” Modifications are currently
being made to minimize the size of the devices and
improve the battery for full implantation in the
body.
“VADs have only been approved for a few
years. From the patient and FDA perspective, it’s still
a relatively new technology,” says Aurand. Initially a
temporary fix until a donor was available, the devices
are now also available to patients who have severe heart
failure but don’t need a transplant.
External
defibrillators are tools that can be found in airports,
hospitals, and schools. Last year, FDA approved the
first automated external defibrillator for
over-the-counter sale. The biggest battle this
technology will face is whether communities and
businesses see an advantage despite the comparatively
high cost.
Artificial Bone and Skin Grafts: New
Materials Provide Better Scaffolding
Erik Swain
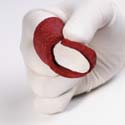 |
 |
| The Vitoss bone fillers, made
by Orthovita Inc., mimic the chemical structure
and composition of human bones, enabling ingrowth
of the host bone. |
Over the past several years, bone and skin grafts
have come a long way. In many cases, they are using
natural substances to be more compatible with the body.
A number of applications have resulted from advances in
surface technologies. For example, coatings that attract
proteins can help the body accept the new graft. They
may also contain calcium and other substances to foster
regrowth. The end result is that grafts look better,
feel better, and react to the body better than was even
conceived of only a few years ago.
Some surgeons
prefer to use the patient’s own bone or skin in
procedures. However, for some reconstructive cases
involving serious trauma, that proves impossible. And to
reduce pain and complications, some clinicians would
rather not harvest a patient’s bone or skin.
In
come cases, advancement has come through tissue
engineering. One example is the work of
Orthovita Inc. (Malvern, PA), which has
developed several synthetic biologically active bone
fillers. Being biologically active is key: it can allow
for stimulation of bone growth or fusion. One product,
Vitoss, uses calcium phosphate to allow resorption, cell
seeding, and ingrowth of host bone. It can do this
because it mimics the chemical structure and composition
of human cancellous bone.
Another company,
Osteotech Inc. (Eatontown, NJ),
specializes in regrowth of human bone and tissue, often
for transplantation procedures. Among its advances is a
demineralized bone matrix that is made from bone fibers
and combined with surface modification techniques to
maximize acceptance by the body. Regeneration comes from
partnering biologically active tissue forms with
nonbiologically active technologies.
On the skin
graft side, Integra Lifesciences Corp.
(Plainsboro, NJ) makes a dermal regeneration template
that entices skin cells to regenerate for burn and
reconstructive-surgery patients. It contains a
replacement layer made from collagen and
glycosaminoglycan and a temporary epidermal substitute
made from silicone to control moisture
loss.
These and other efforts are blurring the
boundaries between the natural and the synthetic.
Artificial Orthopedic Disks: Flexible Disks
Imitate Vertebrae
Maria Fontanazza
 |
 |
Top: DePuy’s Charité artificial
disk is used in patients who suffer degenerative
disk
disease. Bottom: The Charité disk in the
spine. |
Innovative design combined with breakthrough clinical
benefits means artificial spinal disks are a
transformational technology. Although the devices have
existed for about 20 years, their entry last year into
the U.S. market offers more back-pain sufferers an
alternative to spinal fusion surgery.
“The
artificial disk has the potential to actually change the
world of the spine, much like the world of the knee and
hip has been changed by implants developed over many
decades,” says Greg Aurand, senior medical devices
analyst at Zacks Investment Research
Inc. (Chicago).
The orthopedic devices
will affect both the patient population and the spinal
market. Global revenues for spine arthroplasty were $75
million last year, according to Anthony Viscogliosi,
principal at Viscogliosi Bros. LLC (New
York City). He predicts that by 2010, the market for
nonfusion spine technology will reach more than $10
billion, the largest component of which will be disk
replacement.
This segment has excited the
industry’s major players in orthopedics, so much so that
it has generated more than $1 billion in acquisitions.
That, among other things, has spurred business creation:
Viscogliosi Bros. counts about 130 start-ups in the
spine field, almost all in the nonfusion arena. At least
30 companies are attempting to develop disk
technologies.
So far, DePuy Spine
Inc.’s (Raynham, MA) Charité is the only
FDA-approved artificial disk. It is used in patients who
suffer from degenerative disk disease at the lowest
segments of the lumbar spine and who have not been
helped by at least six months of nonsurgical treatment.
The high-density plastic sliding core, made of
medical-grade plastic, is placed between and supported
by two metallic endplates. These endplates are made of
medical-grade cobalt-chromium alloy and have small teeth
that fasten to the adjacent vertebrae. The flexibility
of the disk imitates the spine’s natural movement to
reduce further weakening of nearby spinal
levels.
Implantable disks pose a threat to spinal
fusion, a procedure that can limit mobility and increase
pain over time. Spinal surgeons’ adoption of the disks
remains to be seen, however.
“The artificial
disks, at least the Charité in particular, go through
the front of the body,” says Aurand. “Like many
minimally invasive surgical procedures, it requires
special training and use of new techniques and tools to
get it up to speed.”
But if artificial disks
catch on, they could significantly affect a spine
surgeon’s business. “If one spine surgeon is not
educated and trained on this technology, another one
down the street will be,” says Viscogliosi. “Patients
will go to the surgeon who can provide a solution for
their pain.”
What lies ahead? Perhaps complete
implantation, says Viscogliosi. “It will usher in a new
wave of thinking to preserve motion in the spine,
setting the stage for rapid growth in other areas of
spinal nonfusion,” he says. These areas include disk
nucleus replacement for low back pain and nonfusion
surgical solutions for back pathologies, like adolescent
scoliosis.
Cervical disks are also currently
being developed and tested for use in the upper portion
of the back. If investigational trials are successful,
they could be available in a few years, says Aurand.
Nucleic Acid–Based IVDs: Diagnoses in a Day,
Not Weeks
Brendan Gill
 |
| The Rapid Capture System from
Digene (Gaithersburg, MD) can process 352 patient
specimens in 61¼2
hours. |
The potential of nucleic acid–based IVDs (NA IVDs)
has captured the imagination of the public and industry
alike. The technology’s allure—to help doctors diagnose
or prescribe medicine or treatment based on a patient’s
DNA—is the stuff of sci-fi movies. Although truly
personalized medicine may be off in the distance, NA
IVDs are significantly changing the world of diagnostics
today.
The technology’s applications are in the
hundreds, but infectious-disease control has benefited
especially from NA IVDs. Products like the Procleix WNV
Assay from Gen-Probe Inc. (San Diego)
have improved blood screening for the West Nile virus.
The assays, made available two years ago, are used to
screen more than 80% of the U.S. blood supply.
“NA IVDs are far more sensitive and, in many
cases, faster, than older diagnostic technologies,” says
Dan Kolk, associate director of development at
Gen-Probe. “This leads to detection earlier in the
disease cycle. Most NA IVD tests can be performed in
less than a day, whereas older tests took days, and, in
some cases, weeks.”
NA IVDs are also improving
sexually transmitted disease testing. Approximately 4
million cases of gonorrhea and chlamydia occur in the
United States each year. NA IVDs are helping health
professionals obtain faster, more-accurate diagnoses
with better techniques. The test’s sensitivity and
specificity have been improved through the development
of target- and signal-amplification methods. Also, tests
based on enzymatic target amplification have enlarged
target molecules to be large enough for detection with
reporter systems.
Pharmacogenomic products have
also reduced the trial-and-error risk of drug
prescriptions. Products such as Herceptin, from
Genentech Inc. (San Francisco), are
effective only if breast cancer cells have extra copies
of the Her-2/neu protein. Better-targeted drugs have cut
down on adverse reactions, which contribute to millions
of hospitalizations a year.
NA IVDs will
continue to improve on the strides made so far. Truly
personalized medicine is still in the future, but NA
IVDs will give a clearer picture of what that future
looks like.
Medical Lasers: The Wavelength of the
Future
Maria Fontanazza
 |
| IntraLase Corp. uses a
computer-guided laser to reduce the risk of cornea
cutting. |
As the possibilities for laser technology continue to
grow, it’s not unrealistic to say that someday lasers
will be used in nearly every surgical
procedure.
“For applications in laser surgery,
we’re only scratching the surface,” says Elizabeth
Tanzi, codirector of laser surgery at the
Washington Institute of Dermatologic Laser
Surgery (Washington, DC). “Laser and
light-source treatments are improving and expanding
every year.” Lasers are widely used in operating rooms
and outpatient facilities.
“The recovery for
patients is usually far less than cold-steel surgery,”
says Tanzi, who uses more than 25 different lasers to
treat skin conditions. “Over the past five years, we’ve
noticed improvements in noninvasive treatments.”
One very promising application for lasers is in
photodynamic therapy for skin cancer, says Tanzi.
Following the injection of a photosensitizer, a red
light is aimed at the cancerous area, and it shrinks or
destroys the tumor. Ongoing research is studying ways to
improve photodynamic therapy for use fighting
cancer.
 |
LumaCare (Newport Beach, CA)
uses
photodynamic therapy to treat tissue
diseases and
disorders. |
In ophthalmology, it’s eliminating the need to wear
glasses. LASIK surgery is a procedure that uses an
excimer laser to permanently change the cornea’s shape.
In recent years, it has become the primary method used
to treat myopia in the United States.
“We’re
getting to the point where LASIK is a much better and
safer procedure,” says Eliot Lazar, MD, president of
ElCon Medical Consulting (Buffalo, NY).
“It will continue to evolve over time.”
“It’s a
pretty competitive market, and it’s still expanding,”
says Greg Aurand, senior medical devices analyst at
Zacks Investment Research Inc.
(Chicago). “As people become more accustomed to the
procedure, and you have some decent data that say it’s
not a high-risk venture, you’re going to get market
growth out of it.”
IntraLase
Corp. (Irvine, CA) took the procedure further
and replaced the handheld knife used in the first step
with a computer-guided femtosecond laser. “IntraLase’s
process, in theory, should cut down the risk of error in
hand cutting the cornea, and using a laser to do it
should provide a better patient benefit,” says Aurand.
Long-term studies are still needed to assess its
results.
Progress in surgery and instruments will
affect the innovative course of lasers. Applications
will continue to expand and will become more prominent
in treating severe conditions.
Medical Imaging Technology: The Value Beneath
the Surface
Heather Thompson
 |
The Volume CT scanner from
Siemens can present
unprecedented 3-D
images. |
The old cliché of a picture being worth a thousand
words doesn’t even begin to describe advances in medical
imaging. The technology has been a giant since it was
invented in the early 1900s. And while those first basic
x-rays were impressive, today’s imaging devices have
reduced process time from days to mere seconds and
transformed the images from shadowy gray figures into
fully rendered photos or videos. The images also come in
3-D and full color.
Imaging devices are often
broken into five modalities: x-ray, computed tomography
(CT), magnetic resonance (MR), ultrasound, and nuclear
medicine (or radionuclide scanning).
Although
all are used for depicting internal body parts, they
each have a forte. Ultrasound, for example, is
noninvasive and suited for soft-tissue organs that may
be sensitive to radiation. MR is used for many types of
soft-tissue imaging and presents sharp-contrast detail
between different tissues with very similar densities.
CT provides detailed cross-sectional images and
diagnostic information for almost all body structures.
Spiral CT enables the acquisition of data for 3-D
reconstruction. The granddaddy of imaging, the x-ray, is
still used today because it is a fast and easy way to
assess bone and tissue. Nuclear medicine images show
less detail than other types of imaging, but they convey
the function of an organ based on how much radiation the
organ absorbs.
Scanners can be used to view any
part of the body, and they are now branching out to
other medical disciplines. Picture archival and
communications systems (PACS) are gaining attention
because they provide storage, transmission, and display
in real time. They can even perform calculations such as
counting plaque deposits or measuring bone loss.
 |
The Siemens Axiom Artis
enables real-time communication
outside the
imaging room. |
Manufacturers are also improving the speed and
capabilities of imaging technology. Recently,
Siemens Medical Solutions (Malvern, PA)
and Massachusetts General Hospital
(Boston) built a prototype for a volume CT scanner.
The area-detector–based scanner uses the Somatom
Sensation CT gantry and has a 2-D digital flat-panel
detector technology. The new system features volume
coverage of 18 cm3 with up to 768 CT slices per
rotation.
Although it is not yet ready for human
use, the “area-detector CT technology has the potential
to introduce important future pathways of CT
applications,” says Bernd Ohnesorge, PhD, vice president
of global CT marketing and sales at Siemens Medical
Solutions.
According to the company, a medical
professional using volume CT could directly see the
trabecular structure of bone or the dynamic contrast
uptake of tumor tissue. It could help identify the
composition of atherosclerotic plaque in the vascular
system and coronary arteries and spotting
microcirculation of the cardiac muscle.
As the
technology gets more and more sophisticated, the price
of imaging devices will rise. Medical imaging scanners
and auxiliary products are expected to reach $10.4
billion by 2009 in the United States.
However,
in the end, these imaging devices are so popular because
they save money, time, and, of course, patients.
Replacing biopsies with images reduces patient
discomfort, cost, and time. And in some cases, using
imaging can eliminate surgeries and patient hospital
stays altogether. Those savings are hard to put a price
on.
Wireless Technology: Hospitals and
Homes Go Unplugged
Heather Thompson
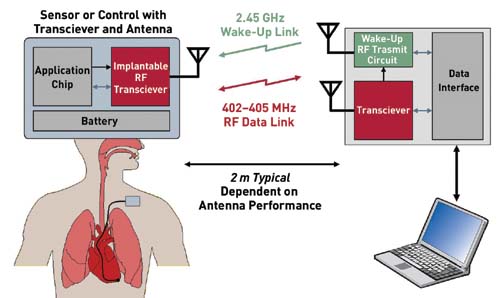 |
| Once implanted, transceiver
chips like the one from Zarlink must conserve
energy and alert hospital staff of critical
changes in the patient (click to
enlarge). |
Although consumers have had access to wireless
telecommunications devices for several years, hospitals
are only now beginning to use the technology for data
capture. But for hospitals, wireless applications are
more than just a convenience. A mobile connection to
data records and real-time updateable information could
reduce errors, which could mean the difference between
life and death.
Wireless technologies employ
data communication systems that link together various
users. A wireless local area network (WLAN) can span an
office, a building, or even a medical campus.
The technology can be simply explained, although
it’s not as simple in practice. Radio and infrared
electromagnetic waves transmit the data at a defined
frequency. The preferred frequency for medical devices
is 402–405 MHz. Base stations integrate data into the
facilities’ LAN. They often link to access points or
antennas that can be placed strategically to extend the
distance between a base station and the end users. The
end unit is usually a receiver. It can be almost
anything that collects crucial information—a nurse’s
personal digital assistant, a radio-frequency
identification reader that tracks blood for transfusion,
or a patient-monitoring system such as an
electrocardiograph machine.
As the technology
becomes more sophisticated, companies are fine-tuning
ways to use wireless. Companies such as Hospira
(New Orleans) are building customizable
wireless software into products. For example, the
company’s MedNet software has been packaged with
drug-delivery systems to help reduce errors.
 |
| The Plum A+ wireless system
from Hospira defines dose limits and tracks IV
drug delivery. |
Transceivers (electronics that both receive and
transmit data) are also being incorporated into medical
implants. Even after they are implanted, the devices can
transmit performance data on patient health.
Zarlink Semiconductor (Ottawa, ON,
Canada) has introduced such a wireless transceiver chip
designed specifically to meet medical implant
communication service (MICS) standards.
Edward
Goffin, communications manager for Zarlink, believes the
device will be used at first with pacemakers and
defibrillators, but there is potential for other
devices, too. “We have a customer who might want to use
it for blood glucose meters. Then doctors could implant
both the sensor and the insulin into the body and have
the whole thing run by the chip.”
Zarlink’s chip
transfers data at a rate of up to 800 Kb/sec. “It’s
pretty dramatic,” says Goffin. “That’s like going from a
dial-up to wireless broadband Internet connection.”
The transceiver operates up to 2 m away from the
base station, as opposed to earlier models that needed
to be within inches of the base station to work
properly. The base station is about the size of a
cellular phone. “Patients could have the base station by
their bedside or clipped onto their belt,” Goffin says.
It connects directly to the necessary hospital computer
for monitoring.
The devices can be integrated
into imaging centers, laboratories, operating rooms, and
now patients. Nearly every point of patient care, and
some places patients never see (e.g., administration
offices), will eventually benefit from wireless
technology.
Computer-Assisted Surgery: The Digital OR
Brendan Gill
 |
| Brainlab uses CT and
fluoroscopic images to navigate instruments during
spine surgery. |
Computer assisted-surgery (CAS) is to the surgeon
what GPS technology is to the wandering driver. Both
technologies have revolutionized navigation, one inside
the human body and one on the highway.
CAS, or
navigation surgery, uses an image-guided camera and a
computer to guide a surgeon’s hands. The camera receives
signals from a surgeon’s instruments and then projects
the image onto a monitor. This enables a surgeon greater
precision and control and results in less trauma and
shorter recovery times for patients.
CAS
“improves surgery in three key areas: it facilitates
more-accurate bone cuts and component alignment, reduces
outliers, and enables less-invasive techniques,” says
Cameron Georges, director of sales for orthopedics
at Brainlab (Westchester, IL). “It also
provides quantitative information relative to key
variables like ligament balancing, leg length, and
range of motion.”
Computers in the operating room
have required that medical devices adapt to the
higher-tech environment. Companies such as Brainlab and
DePuy, Biomet Inc., and Zimmer Inc., all based in
Warsaw, IN, are developing medical devices to meet the
needs of computer-assisted ORs.
New devices
include reference arrays and clip-on adapters. Reference
arrays, or small, reflective spheres that transmit
information, must be added to medical instruments to be
picked up by CAS cameras. Existing instruments are
fitted to CAS by using plane adapters that fit in
cutting slots or by the use of clip-on adapters, says
Georges.
 |
The Vector Vision software
from Brainlab can track surgical
instruments
on a computer screen. |
CAS has significantly affected minimally invasive
surgery. With CAS, incisions can be smaller and can be
made with greater accuracy. However, with smaller
incisions comes less visibility for the surgeon.
“The biggest thing is visibility,” says Ryan
Shoenefeld, product development engineer in digital
surgery at Biomet Inc. “But with CAS, you have the
ability to track instruments inside the body with good
accuracy. If you’re going to put a hip through a 3–4-in.
incision, the system will tell you if you’re putting an
acetabular cup in 15° of anteversion. You have the
ability to improve outcomes of minimally invasive
surgery.”
DePuy is also developing smart
implants that can communicate with CAS systems to make
up for the surgeon’s reduced visibility. The implants
have embedded microchips that communicate with CAS
systems. The microchips provide information on whether
the implant has moved from the time of surgery, in what
direction, and how far. Also in the works at DePuy is a
detector that collects information from the implant.
Copyright ©2005 Medical Device
& Diagnostic Industry
Comments about this article?
Post
them in our Members'
Discussion Forums. |
|